Research & Development
Vocal fold scar
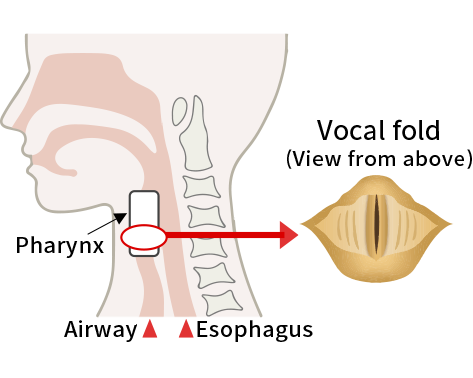
What is vocal fold scar?
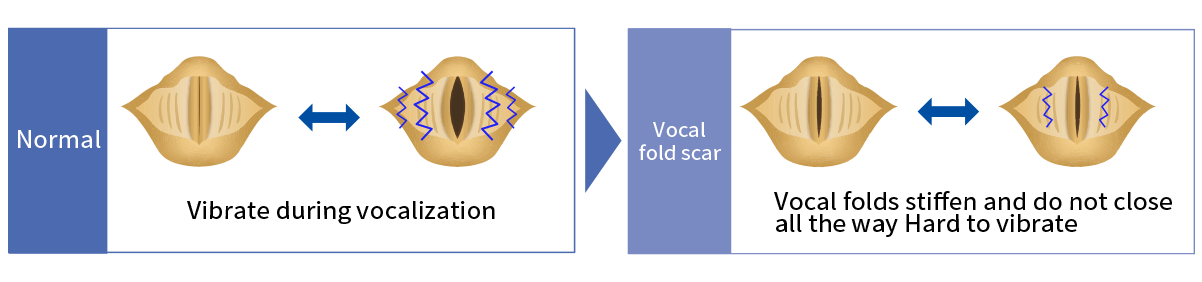
Vocal fold scar is associated with changes in the vocal fold (fibrosis and scarring), resulting in slow movement and voice disorders such as dysphonia. Although its pathogenesis is not clear, it often occurs after vocal cord trauma, inflammation, or surgery. Based on the results of a small-scale epidemiological study, it has been estimated that there are 3,000 to 12,000 patients with vocal fold scar in Japan. To date, there is no effective treatment for this condition. Symptomatic treatment is the standard approach which involves rehabilitation with vocal training and surgery to adjust the position of the vocal cord.
Characteristics of the vocal fold
- Vocal folds vibrate 200 to 300 times per second to produce sound
- Layered structure consisting of mucous membrane on the surface and muscles and ligaments making up the internal part
Characteristics of the disease
- Chronic intractable disease, congenital or acquired (due to inflammation or trauma)
- Vocal fold mucosa harden and degenerate due to fibrosis, resulting in difficulties using the voice
- Number of patients*: 3,000 to 12,000 in Japan, 30,000 to 120,000 in developed nations
- There is no effective treatment
-
- * Source: Koichi Tsunoda,
- Research to Formulate Guidelines for Establishment and Standardization of Diagnosis and Treatment of Vocal Fold Abnormalities.
The Company’s estimates are based on the FY2009 Summary and Co-investigator Research Report, Research for Overcoming Intractable Diseases, MHLW Grant-in-Aid for Scientific Research, and the “World Population Prospects” by the Statistics Bureau of the Ministry of Internal Affairs and Communications.
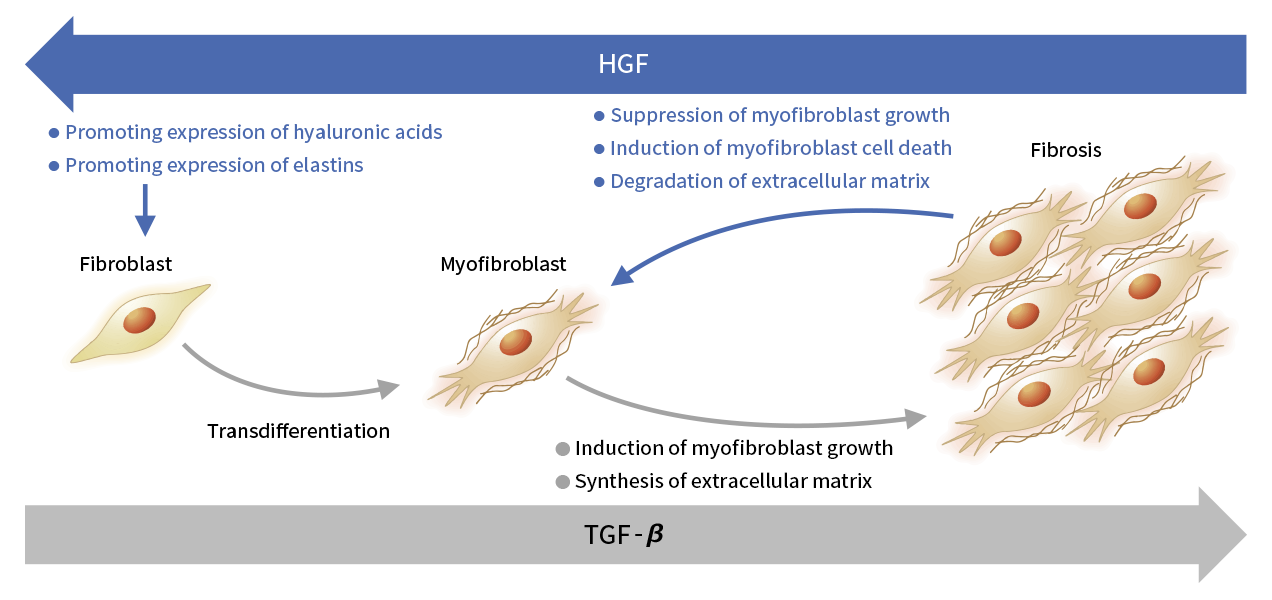
Mechanism of action of HGF in vocal fold scar
In addition to other biological activities, HGF mediates the antifibrotic effect that can be used to treat vocal fold scar. In collaborative work with the Department of Otolaryngology, Head and Neck Surgery, Graduate School of Medicine, Kyoto University, and the Foundation for Biomedical Research and Innovation at Kobe, we injected recombinant human HGF into the vocal cords of an animal model of vocal fold scar and confirmed improvement in vocal cord function. We then conducted additional preclinical studies involving vocal cord injection and progressed to the clinical trial stage.
Summary of Phase I/II study in vocal fold scar (investigator-initiated clinical trial)
The Phase I/II study (investigator-initiated clinical trial) was conducted as an open-label dose-escalation study in patients with vocal fold scar. HGF was administered topically into the vocal fold mucosa bilaterally (once a week x 5 times). The primary endpoint was safety, and the results of the study confirmed the study drug was well tolerated and did not cause serious adverse reactions. In addition, the secondary endpoints showed efficacy trends and provided insights on the evaluation timing of efficacy. The results of this study have been published in the international medical journal “Journal of Tissue Engineering and Regenerative Medicine” (Hirano et al, 2018.). Once a POC is obtained with HGF for the treatment of vocal fold scar, the drug discovery concept based on the anti-fibrotic effect of HGF will be confirmed. It will pave the way for a wider application of HGF to other chronic diseases caused by fibrosis (chronic renal failure, liver cirrhosis, pulmonary fibrosis, etc.).
| Study design | Open labelled, dose escalation study | |
|---|---|---|
| Patient population | Patients with vocal fold scar, patient’s age was ≥20 years and ≤65 years | |
| Dosage and administration | 1, 3, 10 µg, unilaterally injected into the vocal fold, once a week, 4 times (same dosage was injected into both side vocal folds) | |
| Primary endpoint | Evaluation criteria | Safety |
| Results | Incidents of hyperemia of the vocal folds were observed, then all cases recovered. No other serious adverse event was observed. | |
| Secondary endpoint | Evaluation criteria | Phonatory outcomes evaluated by examining the improvement in each phonatory parameter |
| Results | 3 of the 5 phonatory outcomes indicated tendency of improvement | |
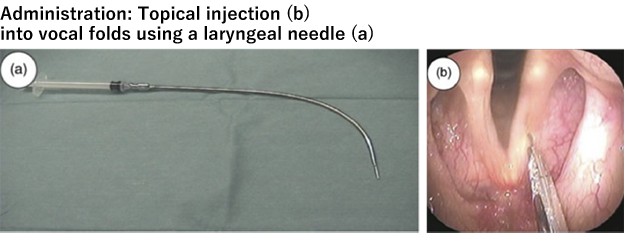
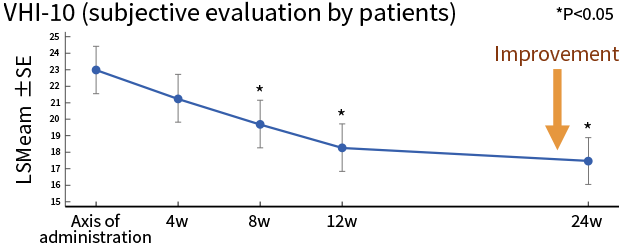
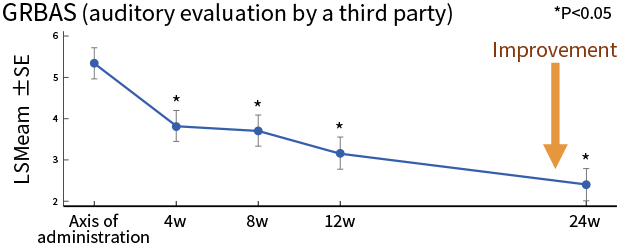
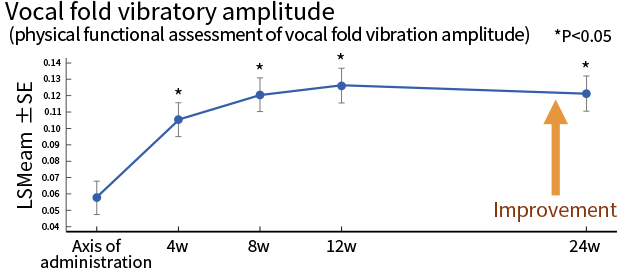
*Source: Hirano et al, J Tissue Eng Regen Med. 2018
Phase III study in vocal fold scar (final stage of drug development)
Phase III study in patients with vocal fold scar
Based on the results of the Phase I/II study, a pivotal Phase III study was initiated as a multicenter, randomized, placebo-controlled, double-blind, parallel-group comparative study of intracordal administration of KP-100LI in patients with vocal fold scar. The study aims to confirm the safety and efficacy. Patient enrollment has started at University Hospital, Kyoto Prefectural University of Medicine. In addition, we have increased the number of clinical trial sites to eight.
| Study design | Multicenter,randomized study |
|---|---|
| Enrollment | 62 subjects (HGF and placebo groups, 31 subjects each group) |
| Subject population | Patients with vocal fold scar (including vocal fold sulcus)、age: 18-75 |
| Dosage and administration | Double-blind period: intracordal injection (four times weekly)、observation period:24weeks Open-label extension period: HGF administered to patients who wish to continue treatment (four times weekly)、observation period:24weeks |
| Primary endpoint | Improvement in VHI-10* score at week 24 of double-blind observation period |
| Investigation sites | Kyoto Prefectural University of Medicine Hospital, Kurume University Hospital, Tohoku University Hospital, Kawasaki Medical University Hospital, Sanno Medical Center, Fujita Health University Hospital, Fukuoka Sanno Hospital, and 1 other clinical trial site |
Development and manufacturing of commercial formulation
- Development of a small dose formulation for commercial use
- Commercial scale manufacturing and relevant studies
-
- *
- The dosage for vocal fold scar is less than one-tenth of that for neurological diseases. Although the same formulation used in clinical trials for acute phase spinal cord injury and ALS is used in diluted form in the clinical trial for vocal fold scar, a small dose formulation is necessary for commercialization.

References
- Mizuta M, Hirano S, Ohno S, Kanemaru S, Nakamura T, Ito J. Restoration of scarred vocal folds using 5 amino acid-deleted type hepatocyte growth factor. Laryngoscope. 2014; 124: E81-86.
- Hirano S, Kawamoto A, Tateya I, Mizuta M, Kishimoto Y, Hiwatashi N, Kawai Y, Tsuji T, Suzuki R, Kaneko M, Naito Y, Kagimura T, Nakamura T, Kanemaru SI. A phase I/II exploratory clinical trial for intracordal injection of recombinant hepatocyte growth factor for vocal fold scar and sulcus. J Tissue Eng Regen Med. 2018 12:1031-1038.