Research & Development
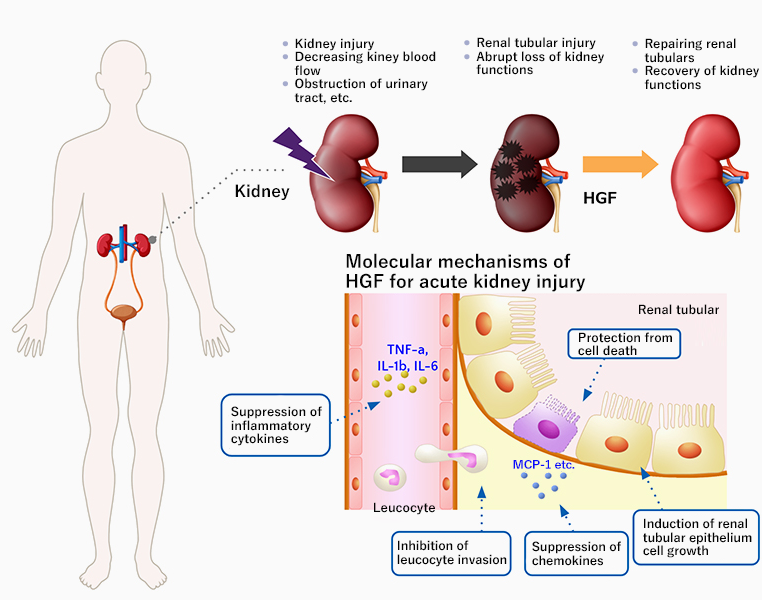
Acute kidney injury
What is acute kidney injury?
Acute kidney injury is characterized by a rapid decline in renal function over a short period of time (hours to days) due to conditions such as kidney damage, inadequate blood supply to the kidneys, and urinary tract obstruction, resulting in the inability of the kidney to excrete fluids and waste products in the urine and maintain the balance of fluids and salts in the body. Reportedly, acute kidney injury can occur in 5% to 7% of inpatients and 20% to 25% of patients in intensive care units. There are about 80,000 new cases of acute kidney injuries in Japan every year. Emergency care is required for serious cases and is associated with high mortality. The cause of acute kidney injury is often unidentified, as a number of factors are involved in its development, and no effective treatment has been established yet.
Mechanism of action of HGF in acute kidney injury
As HGF protects kidney cells and promotes cell growth, it has a potential to be an effective drug for treating acute kidney injury. We conducted early clinical trials in the U.S. with the support of a nephrology clinic (Rogosin Institute). As the objective of the Phase Ia and Ib studies was to confirm the safety and pharmacokinetics of the intravenous administration of recombinant human HGF, we considered it inappropriate to enroll acute kidney injury patients whose condition is usually unstable. Thus, chronic kidney disease patients with relatively stable condition were enrolled in the studies. We received fast track designation from the U.S. Food and Drug Administration to conduct this study, which is a program to expedite the review of new drugs with immediate medical needs.
Summary of Phase I studies in chronic kidney disease
Based on the findings of this study, the next phase of clinical trials in patients with acute kidney injury is being designed. At the same time, we are also developing an additional Phase I study to be conducted in Japan (a small-scale study to determine the maximum tolerated dose in Japanese patients). Specifically, to develop a protocol for patients with acute kidney injury, we are continuing to interview medical experts, review other companies’ clinical trials, and examine biomarkers. Currently, the next phase of clinical trial is expected to be a relatively large, placebo-controlled, double-blind comparative study. To implement this clinical trial, we are first looking to secure funds by partnering with pharmaceutical companies.
As the highest level of systemic absorption of the test drug can be achieved by intravenous injection, safety issues are likely to occur using this route of administration. We have procured important information for the development of other routes of administration as the safety of the drug was confirmed in these studies. Intravenous injection can easily be expanded for applications in various diseases. We will continue development on this front while identifying diseases other than acute kidney injury for which the safety and efficacy of the drug can be ensured.
| Study design | Phase Ia: open labelled, dose escalation study (9 subjects) Phase Ib: placebo controlled, double-blind study (15 subjects) |
|
|---|---|---|
| Patient population | Patients with chronic kidney disease, Patient’s age was ≥18 years and ≤85 years | |
| Dosage and administration | Phase Ia: single intravenous administration for 3 dosage groups Phase Ib: repeated intravenous administration, once a day, 5 times, 2 dosage groups |
|
| Primary endpoint | Evaluation criteria | Safety and tolerability |
| Results | There were no serious adverse events | |
| Secondary endpoint | Evaluation criteria | Pharmacokinetic profile |
| Results | The drug promptly dissipated with single doses, and no accumulation was observed with repeated doses. | |
References
- Matsumoto K, Nakamura T. Hepatocyte growth factor: renotropic role and potential therapeutics for renal diseases. Kidney Int. 2001;59(6):2023-2038
- Kawaida K, Matsumoto K, Shimazu H, Nakamura T. Hepatocyte growth factor revents acute renal failure and accelerates renal regeneration in mice. Proc Natl Acad Sci USA. 1994;91:4357-4361.