Our Business
A late clinical-stage biopharmaceutical company that aims at the practical application of HGF-based medicines
Kringle Pharma was established as a university spin-off bio-venture dedicated to the R&D of therapeutic agents for intractable diseases, which refers to rare diseases that have unknown or poorly understood causes and no established treatment methods, and that negatively impact daily life for an extended time.
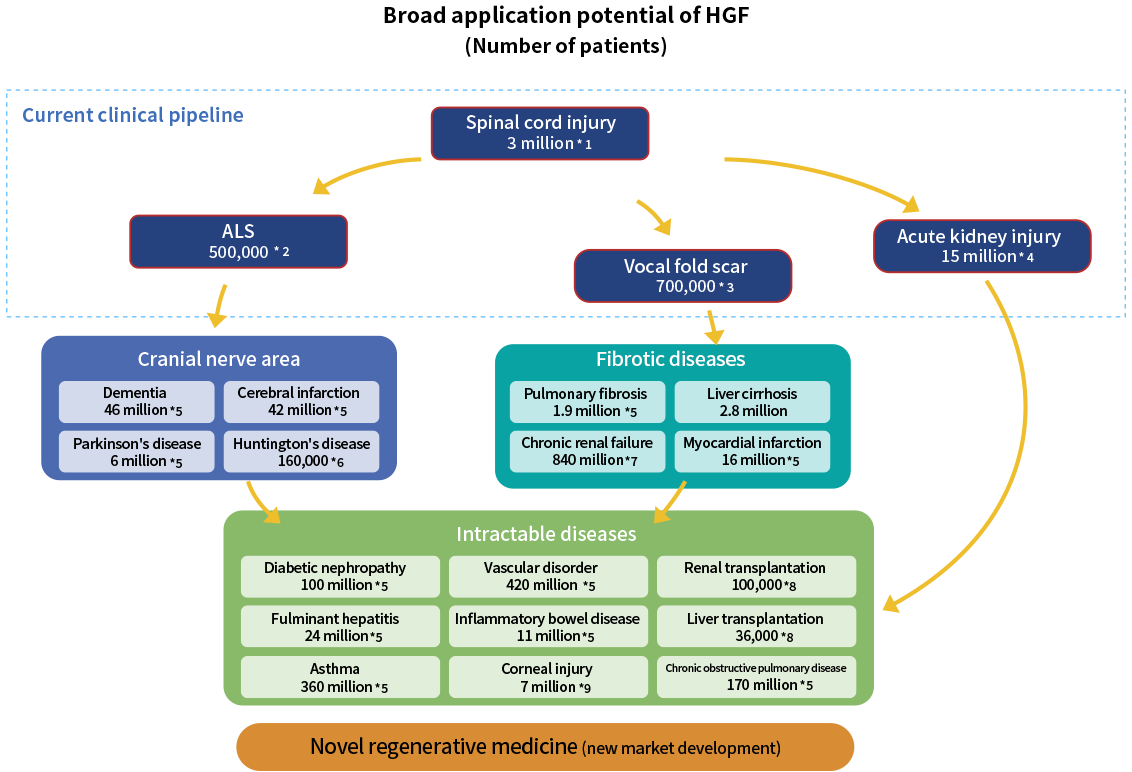
We have already succeeded in producing pharmaceutical-grade recombinant human HGF and are conducting clinical trials for acute phase spinal cord injury, vocal fold scar, amyotrophic lateral sclerosis (ALS), and acute kidney injury. Starting with these intractable diseases with high unmet medical needs, we will expand the application of HGF-based medicines to other organ diseases.
Diseases with potential clinical application of recombinant human HGF (“HGF protein”)

-
- Kidney
- Acute kidney injury, chronic renal failure, renal transplantation, diabetic nephropathy
-
- Liver
- Acute hepatitis, fulminant hepatitis, liver cirrhosis, biliary atresia, fatty liver, liver transplantation
-
- Heart and blood vessels
- Vascular disease (arteriosclerosis obliterans, vascular restenosis prevention, etc.), myocardial infarction, dilated cardiomyopathy
-
- Nervous system
- ALS, acute phase spinal cord injury, cerebral infarction, Parkinson’s disease, Huntington’s disease, dementia
-
- Lungs and bronchi
- Chronic obstructive pulmonary disease (COPD), pulmonary fibrosis, bronchial asthma
-

- Others
- Skin ulcer, vocal fold scar, inflammatory bowel disease, corneal injury
*Red text indicate target diseases for which we are currently developing drugs
Our strengths
01Late clinical-stage biopharmaceutical
company developing regenerative medicines
- In-house development of HGF-based pharmaceuticals to treat rare diseases
- Multiple late-stage pipelines
- Acute Spinal Cord Injury (Acute SCI)
- Phase III study completed
Orphan drug designation*
* Designation No.: (31 Drug) No. 442 - Vocal fold scar
- Phase III study underway
- ALS
- Phase II study completed
Additional analysis underway - Acute kidney injury
- Phase I study completed
02HGF-based regenerative medicine platform operator
- Recombinant human HGF: First-in-class candidate therapeutics
- Established a system to manufacture and mass produce recombinant human HGF protein for use in pharmaceuticals
- Expanding indications for future growth
Kringle Pharma supplies recombinant human HGF protein as an active pharmaceutical ingredient to Claris Biotherapeutics (US). Claris is conducting a Phase I/II study for the treatment of neurotrophic keratitis.

Our business model
We have adopted a hybrid business model that combines A, B, and C, depending on the characteristics of the target disease and our pharmaceutical partners. In this context, our basic policy is to apply for manufacturing and marketing approval independently, with the aim of reliable practical implementation of the outcomes of clinical trials as pharmaceutical products. We aim to commercialize our clinical-stage pipeline drugs currently undergoing clinical trials through a mix of A and B (in-house development and marketing alliance) in acute phase spinal cord injury and vocal fold scar pipelines, and through B in ALS and acute kidney injury pipelines. Our supply of active pharmaceutical ingredients to Claris Biotherapeutics falls under C.
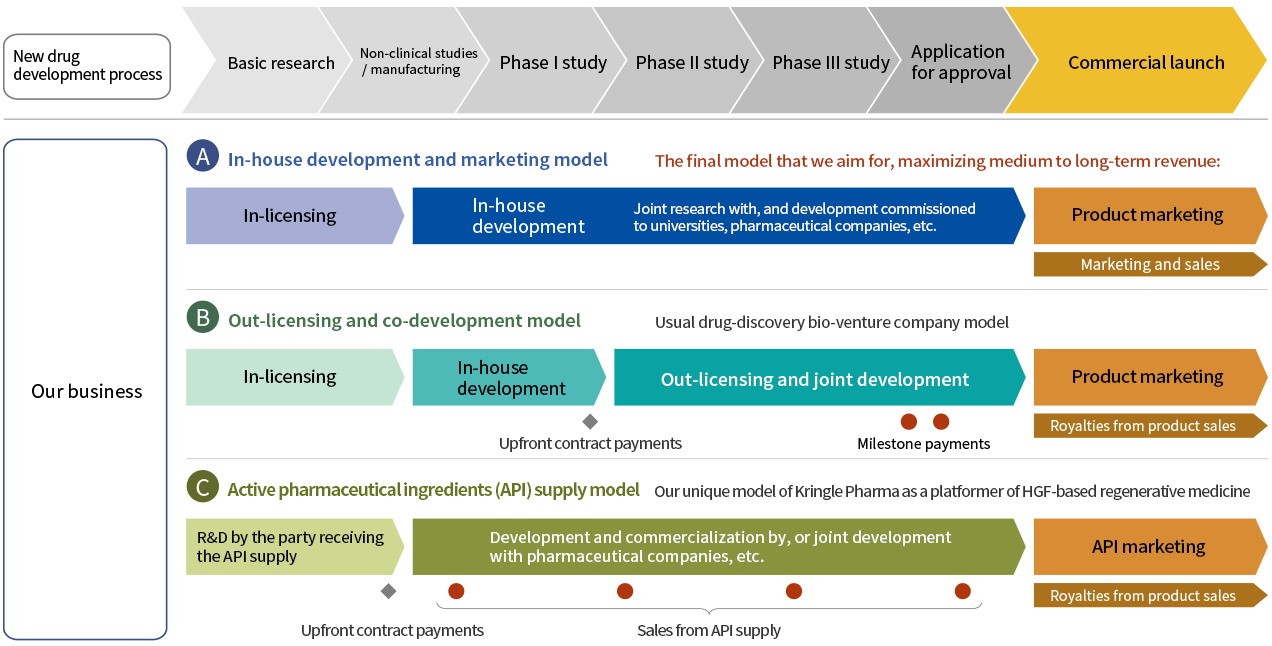
Development must be conducted in accordance with the new drug development process. However, in order to develop new therapeutic medicines for intractable diseases, we go through the following steps:
(1) Basic research
The search for drug candidates requires a long research period, and even if a candidate is found during this stage, the probability of successful development as an approved drug is very low. We conduct basic research to find new indications for recombinant human HGF and new product candidates, and we increase the probability of success of our development by engaging in joint research with academia and gaining access to their specialized knowledge.
(2) Non-clinical studies and manufacturing
We work with contract research organizations (CROs) and contract manufacturing organizations (CMOs) who have the expertise to accelerate nonclinical studies and manufacturing. Because we do not generate revenue that can offset the heavy spending in this development stage, we utilize public funds (grants and subsidies) to reduce the cost burden and mitigate financial risks.
(3) Clinical trials (Phase I, II, and III)
With intractable diseases, because the number of the patients is small and the causes and pathology are often unknown, it is difficult to determine evaluation parameters and select suitable subjects for clinical trials. By collaborating with universities, we have accumulated specialized insights and conducted small-scale clinical trials selecting evaluation endpoints that can increase the probability of success. We outsource clinical trial operations to contract research organizations ensuring quality and speed, while liaison with physicians at specialized and university hospitals enhances the scientific quality of clinical evaluations and data analyses. We also support university hospitals that have public funding and wish to perform investigator-initiated clinical trials, by providing investigational drugs as well as scientific information and knowledge, in exchange for exclusive licenses to make use of research outcomes.
(4) Application for manufacturing and marketing approval
Our basic policy is to apply for manufacturing and marketing approval of drugs independently in order to ensure that the results of clinical trials are implemented as novel therapeutics in society. Drug candidates for the treatment of intractable diseases are eligible to receive an “orphan drug designation” from the Ministry of Health, Labour and Welfare in Japan once a POC (proof of concept) has been demonstrated. This will bring benefits such as subsidies to cover development costs and priority review to accelerate the development process to the regulatory approval. We aim to shorten the regulatory review period and obtain early approval by taking advantage of systems including “Conditional Early Approval System” and the “Pioneer Drug Designation System (formerly the SAKIGAKE Designation System).”
(5) Marketing
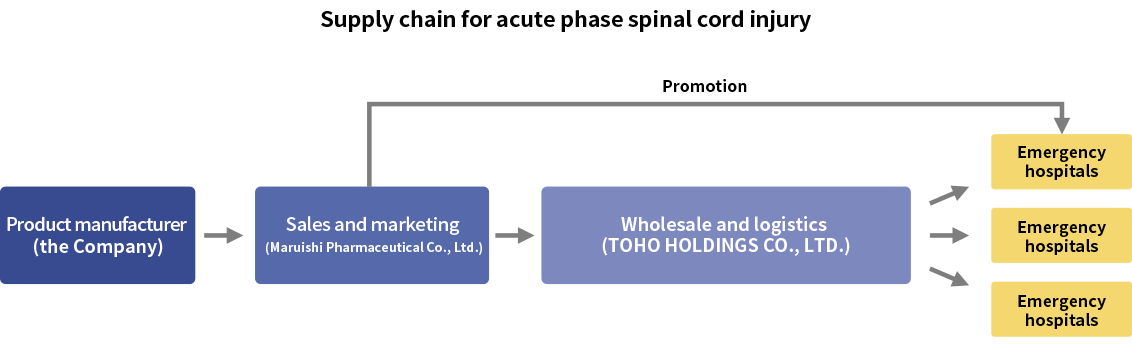
Because intractable diseases are treated mainly at advanced medical institutions, the number of facilities handling the development drug is limited. For this reason, there is no need to establish a large-scale distribution and marketing network as is the case with conventional pharmaceuticals. Moreover, there are few competing drugs in the market as there is virtually no effective treatment, so it is not always necessary to allocate a large amount of resources on sales activities. Therefore, we can simply build a complete supply chain by securing marketing approval on our own and partnering with pharmaceutical companies for marketing and distribution. This approach offers various benefits such as reduced costs associated with sales and marketing and a persistently high gross profit margin. As we do not have an internal marketing organization today for acute phase spinal cord injury pipeline, we partnered with Maruishi Pharmaceutical Co., Ltd. and TOHO HOLDINGS CO., LTD., as shown in the figure below. Maruishi Pharmaceutical Co., Ltd., as a specialty pharmaceutical company* in the field of emergency medicine, has a nationwide sales force that covers most of emergency hospitals in Japan. We believe that we can establish an effective supply chain for sales and marketing of our pipeline drug in acute phase spinal cord injury through this partnership.
(6) Expansion of indications
Although the number of patients with each intractable disease is limited, we believe we can maximize value by running multiple pipelines, exploring opportunities globally and expanding indications to other areas of disease. For example, if HGF is shown to have neuroprotective effect clinically in acute phase spinal cord injury and ALS, we may be able to develop HGF for other neurological disorders in the area of cranial nerves. If HGF demonstrates an antifibrotic effect on vocal fold scar, it may be applicable to other diseases caused by fibrosis. Furthermore, if HGF administered intravenously exhibits efficacy against acute kidney injury, it may be applicable in the treatment of intractable diseases of organs other than the kidney. In short, HGF possesses a variety of biological activities, and once the therapeutic effect on one activity is validated, there is a lot of potential that we can develop HGF to treat diseases in other therapeutic areas. We will grow our business by expanding the indications in our pipeline and continuously generate earnings by supplying APIs to companies that wish to develop HGF-based drug for other therapeutic areas.
Bibliography
-
- *1:
- The global map for traumatic spinal cord injury epidemiology, update 2011, global incidence rate. Spinal Cord (2014) 52, 110–116
-
- *2:
- The epidemiology of amyotrophic lateral sclerosis, Handbook of Clinical Neurology. 2016; 138: 225-38.
-
- *3:
- Calculated by the Company based on the prevalence in Japan as found from the “Research on the establishment of guidelines for the establishment and standardization of diagnostic treatment of vocal fold groove syndrome; FY2009 Summary and Subcommittee Research Report.”
-
- *4:
- KDIGO Clinical Practice Guideline for Acute Kidney Injury: Online Appendices A-F. Kidney Int Suppl 2: 1―132, 2013.
-
- *5:
- Disease and Injury Incidence and Prevalence Collaborators. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016; 388: 545-602 (fulminant hepatitis is listed as acute hepatitis).
-
- *6:
- The incidence and prevalence of Huntington's disease: a systematic review and meta-analysis. 2012 Aug; 27(9): 1083-91.
-
- *7:
- Epidemiology of chronic kidney disease: an update 2022. Kidney International Supplements (2022) 12, 7–11.
-
- *8:
- Current status of transplantation worldwide, “Transplantations” Vol. 56, No. 2
-
- *9:
- Centers for Disease Control and Prevention. Estimated Burden of Keratitis –United States, 2010 (the number of corneal injury patients is indicated as the number of patients suffering from corneal ulcer as an intractable corneal injury).