Research & Development
Acute Spinal Cord Injury (Acute SCI)
What is spinal cord injury?
The spinal cord comprises nerves that connect the brain to the rest of the body and is protected by bones called the spinal column. Spinal cord injury occurs following damage to the spinal cord tissue owing to trauma from an external force, such as accidents and falls. Such injuries can result in loss or impairment of motor or sensory functions to develop motility disorder or sensation disturbance. Reportedly, there are about 17,000 new cases of spinal cord injury per year in the U.S. However, to date, there is no effective treatment for spinal cord damage, and only symptomatic treatment is available, including surgery for a fracture or dislocation of the spinal column that encloses the spinal cord, and rehabilitation for utilizing the remaining nerve functions and conducting activities of daily living.
- Spinal cord injury is typically caused by falls or accidents
New cases*: 5,000 persons/year in Japan, 60,000 persons/year in developed nations - Injury to motor and sensory nerves
- The closer the injury site is to the brain, the more extensive the paralysis
- No effective treatments are available (either in the form of drugs or surgery)
Various types of cell therapies are used in the subacute phase
Only symptomatic treatment, such as painkillers and anti-inflammatory drugs, is available - Rehabilitation: few facilities can admit patients for a long-term hospitalization
- Significant health and economic implications for patients and caregivers
*Sources: Our estimates are based on Hiroaki Sakai et al, “Current situation of medical care for spinal cord injury in Japan” (2010), National Spinal Cord Injury Statistical Center, Facts and Figures at a Glance (2019), and “World Population Trends” by Statistics Bureau, Ministry of Internal Affairs and Communications
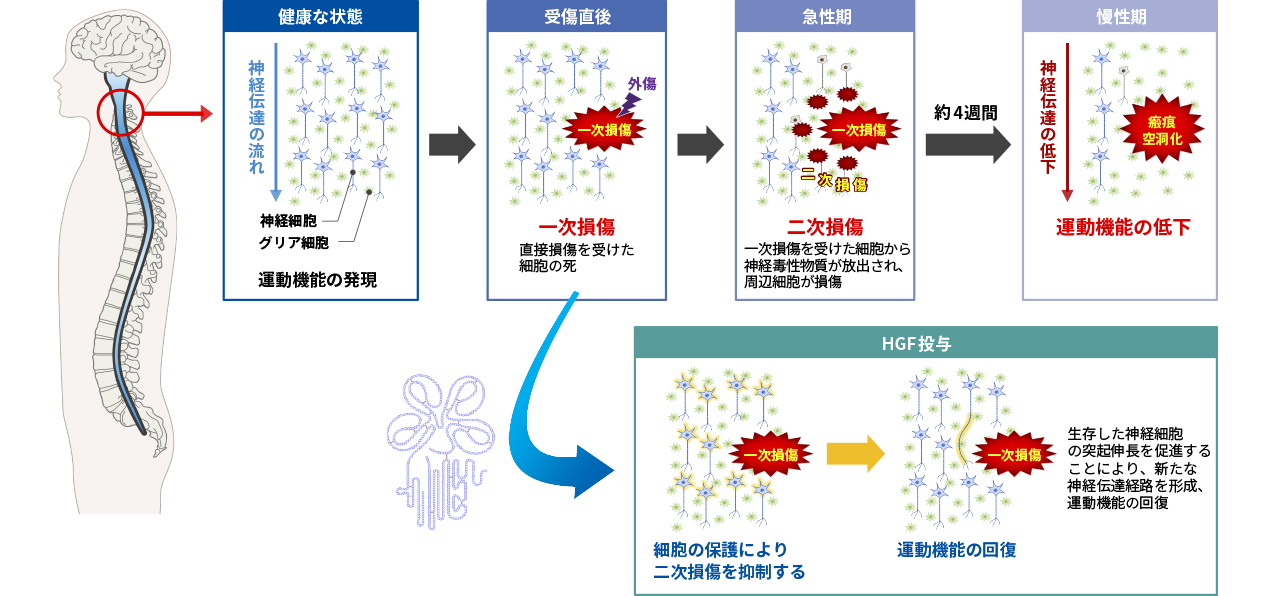
Mechanism of action of HGF in spinal cord injury
HGF is considered to have potential as a novel treatment for spinal cord injury because of its protective effects on nerve cells and its promotion of axonal extension. Spinal cord injury occurs as a result of tissue damage caused by external forces (primary injury) followed by secondary injury, in which the damage spreads to surrounding tissues. In the acute stage of spinal cord injury, it is considered that suppressing this secondary damage is the key to treatment.
In collaboration with the Department of Physiology and Orthopedic Surgery, Keio University School of Medicine, we conducted a study to confirm the pharmacological effects of recombinant human HGF protein using animal models of spinal cord injury, and confirmed its efficacy by evaluating motor function. We then conducted non-clinical studies (toxicity studies, pharmacokinetic studies, etc.) necessary for drug development, and advanced the development to clinical stage.
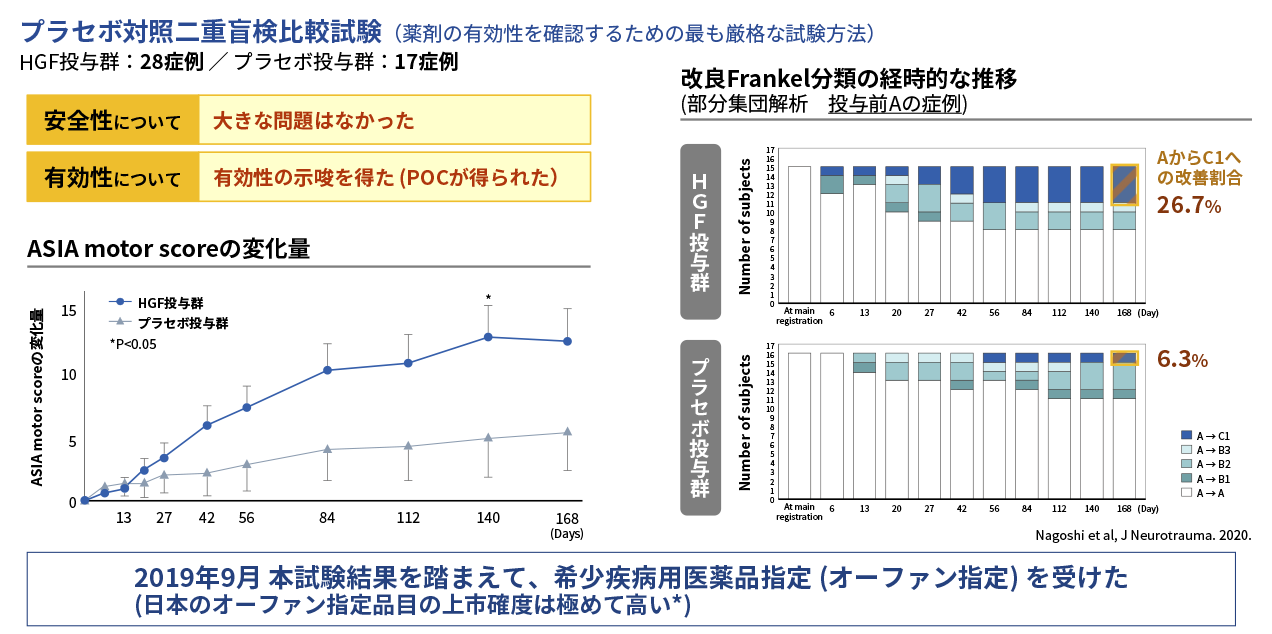
Results of Phase I/II study in acute phase spinal cord injury
The Phase I/II study was conducted as a multicenter, placebo-controlled, double-blind, comparative study to confirm the safety and efficacy of an HGF protein preparation (KP-100IT) in 45 patients with severe acute cervical spinal cord injury (A, B1 and B2 of the modified Frankel classification). KP-100IT or placebo was administered intrathecally (once a week for five weeks) and the patients were followed until six months after injury. The primary endpoint, the change in ASIA motor score at 24 weeks post-injury (Day 168), showed no statistically significant difference between the KP-100IT group and placebo group. On the other hand, secondary endpoints showed a consistent difference in ASIA motor score over time in the KP-100IT group versus the placebo group, with a significant difference at 20 weeks post-injury (Day 140). Furthermore, the percentage of patients who were completely paralyzed with modified Frankel classification score of A at the time of study entry who had improved to C1 incomplete paralysis at the end of observation was 26.7% (4/15 patients) in the KP-100IT group and 6.3% (1/16 patients) in the placebo group, indicating dramatic improvement in the KP-100IT group. The results of this study were published in the international medical journal, Journal of Neurotrauma (Nagoshi et al, 2020.).
*Source: Research on the Current Status of Orphan Drug Designation Requirements in Japan, Research Paper Series No. 70 (2017), Office of Pharmaceutical Industry Research
Phase III study in acute phase spinal cord injury (final stage of drug development)
Based on the results of the Phase I/II study, we received orphan drug designation (orphan designation) in September 2019 and began the Phase III study in-house. The Phase III study is a multicenter, non-randomized study (all subjects will receive KP-100IT) in 25 cases with severe acute phase cervical spinal cord injury (AIS classification A). Dosage and administration is the same as in the previous Phase I/II study, and the primary endpoint is the percentage of patients whose AIS improved to C or better at 6 months after treatment with the investigational drug. Patient enrollment for the Phase III study began in July 2020 and was completed in April 2023.
| Study design | Multicenter, non-randomized study |
|---|---|
| Enrollment | 26 subjects (of whom 25 were evaluated for efficacy) |
| Subject population | Cervical cord injury (AIS score: A), age: 18-89 |
| Dosage and administration | Intrathecal administration (weekly × 5 times from 72 hours after injury), observation period 6 months |
| Primary endpoint | Percentage of patients whose AIS score improved to C or better at six months after treatment with the investigational drug |
| Investigation sites | Five hospitals in Japan |
Concept for an additional study in acute spinal cord injury
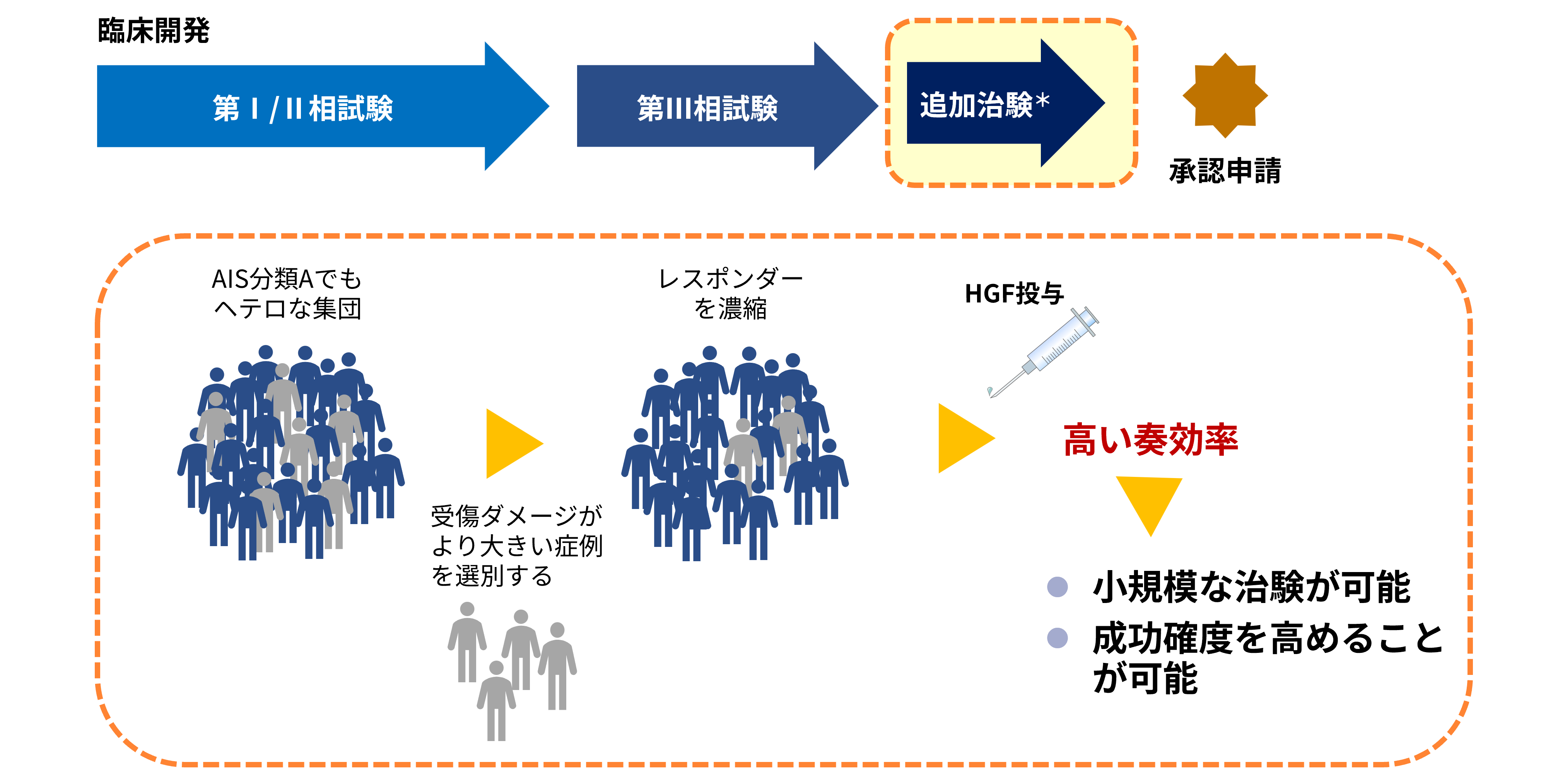
*The outline of the additional clinical study will be disclosed once it is finalized.
As of November, 2025
Acute phase spinal cord injury
Supply chain after regulatory approval
For acute phase spinal cord injury, we plan to file an application and obtain manufacturing and marketing approval by ourselves after the completion of the Phase III study. After acquiring regulatory approval, we will establish the domestic supply chain through alliances with pharmaceutical companies and pharmaceutical wholesalers. In other words, Kringle Pharma is to hold the manufacturing and marketing license, Maruishi Pharmaceutical Co., Ltd. is to market and promote the drug, and TOHO HOLDINGS CO., LTD. to distribute the product to emergency hospitals throughout Japan. Kringle Pharma will receive milestone and royalty payments from Maruishi in addition to the revenue by supplying the product.
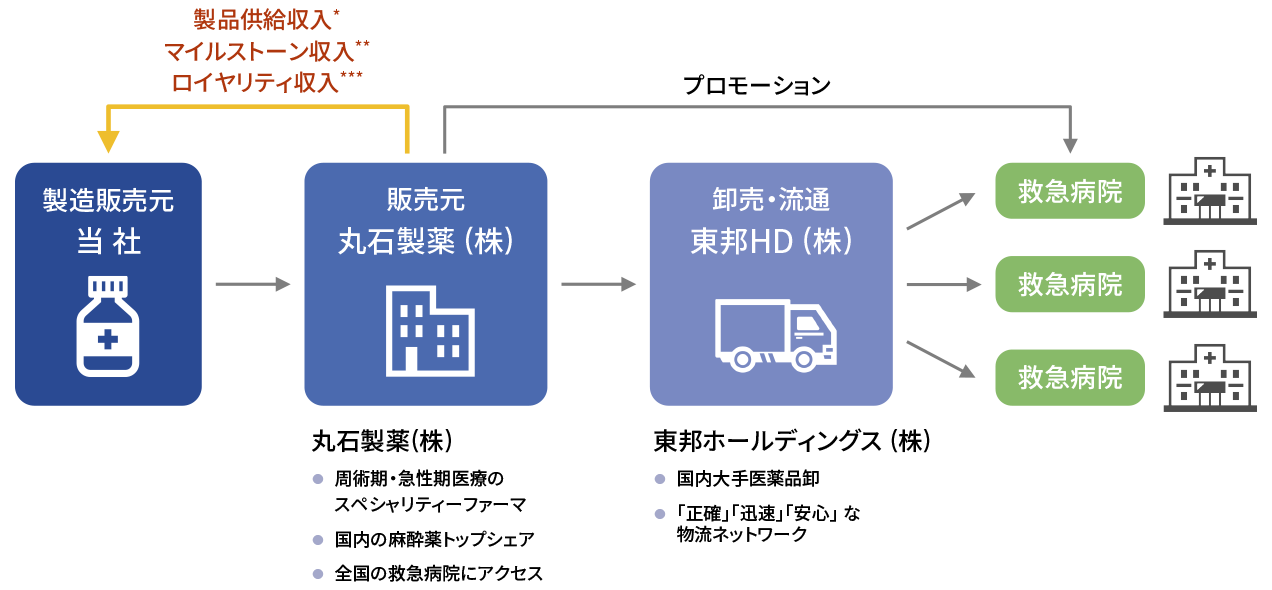
-
- *
- Sold at a unit price obtained by multiplying a certain percentage of the drug price
-
- **
- Development milestone revenue (at the time of application for manufacturing and marketing approval, at the time the drug is listed on the drug price list (if the product is designated under the pioneering drug designation system, a portion is received in advance) and at the time of approval of additional indications), and sales milestone revenue (when sales achieve a certain amount per year)
-
- ***
- After the start of sales, the amount is calculated by multiplying the annual sales by a set rate
Glossary of Terms and References
Term
| Terminology | Definition |
|---|---|
| Modified Frankel classification |
The Frankel classification is a five-level classification of functional disability in quadriplegia, further subdivided according to differences in prognosis. It is classified into 11 levels from A (complete paralysis) to E (normal). |
| ASIA Motor Score | The ASIA Motor Score is an index used by the American Spinal Injury Association to evaluate motor function, consisting of the sum of upper limb (50 points) and lower limb (50 points) motor function scores (in total 100 points). It scores the movement of the major muscles associated with each part of the spinal cord. It is an assessment item for acute phase spinal cord injury widely used due to its ease of implementation and reproducibility. |
| AIS | The ASIA impairment scale is a functional impairment scale for spinal cord injury established by the American Spinal Injury Association. It is classified into five levels from A (complete paralysis), the most severe, to E (normal). |
References
- Kitamura K, Iwanami A, Nakamura M, et al. Hepatocyte growth factor promotes endogenous repair and functional recovery after spinal cord injury. J Neurosci Res 2007; 85:2332-2342.
- Kitamura K, Fujiyoshi K, Yamane J, Toyota F, Hikishima K, Nomura T, Funakoshi H, Nakamura T, Aoki M, Toyama Y, Okano H, Nakamura M. Human hepatocyte growth factor promotes functional recovery in primates after spinal cord injury. PLoS One. 2011; 6: e27706.
- Kitamura K, Nagoshi N, Tsuji O, Matsumoto M, Okano H, Nakamura M. Application of Hepatocyte Growth Factor for Acute Spinal Cord Injury: The Road from Basic Studies to Human Treatment. Int J Mol Sci. 2019 Feb 28;20(5):1054.
- Nagoshi N, Tsuji O, Kitamura K, Suda K, Maeda T, Yato Y, Abe T, Hayata D, Matsumoto M, Okano H, Nakamura M. A phase I/II study for intrathecal administration of recombinant human hepatocyte growth factor in patients with acute spinal cord injury: a double-blind, randomized clinical trial of safety and efficacy.. J Neurotrauma. 2020 Apr 23. doi: 10.1089/neu.2019.6854.